last modified: Tuesday, 26-May-2009 03:45:50 CEST
Document status: construction site
Quartz is the one of the most abundant minerals, second only to feldspars. About 12% of the mass of the earth's crust is made of quartz. Among the reasons for its abundance are:
Once it has formed and appeared at the surface, it will only slowly weather away, if at all. Most of the weathering is due to physical forces: changes in temperature, erosion, cracking by ice-wedges, and grinding.
Under conditions at or near the surface, quartz is chemically more stable than most other minerals, and the 400-500 million years old Herkimer diamonds look just as fresh and new as Alpine rock crystals that formed "just" 10 million years ago. The cactus quartz amethysts from South Africa might be almost 2 billion years old.
Quartz tends to accumulate in deposits of eroded material, both due to its physical and chemical resistance and because it is often formed from silicate minerals during chemical weathering. If you ever traveled through a desert, you might have noted that often the mountains are much darker than the debris surrounding them. The darker components of igneous and metamorphic rocks consist mostly of minerals that easily weather under the influence of carbonic acids, oxygen, and water. Micas and feldspars will slowly decompose and their tiny flakes and grains will be carried away by wind and water.
Not surprisingly, many sedimentary rocks, in particular those that formed on land, contain large amounts of quartz.
Many minerals that formed under high pressure many kilometers below the surface are not stable at normal pressures and will experience a metamorphosis into other minerals. Often they formed under very low oxygen fugacity, a measure of the chemical availability of free oxygen in a rock, and the iron incorporated into minerals is typically present as Fe2+ ions. At the surface the iron gets oxidized to Fe3+, visible as a change of color from a dark gray-green to a brown tone. Iron-bearing silicates will be decomposed and new silicates as well as silica might be formed.
.........
Most of the quartz is contained in granites, a smaller amount in metamorphic rocks (some of which derived from granites), and a small amount in sedimentary rocks. On the other hand, the highest concentrations of quartz can be found in sedimentary and metamorphic rocks as sandstone and quartzite, as well as in alluvial and marine sands and sand dunes. It is clear that the quartz in sand and most sedimentary rocks has been concentrated by erosion and weathering of other rocks, ultimately granites. If we understand the processes that lead to the formation of granites and related rocks, we also understand the abundance of quartz as a rock forming mineral.
Petrology of Quartz
Petrology is the science of the origin and properties of rocks. Strictly spoken, there is no "petrology of quartz", as quartz is a mineral, not a rock. But quartz is an important constituent of many rocks and also plays an important role in their formation. Quartz is said to be very abundant and widespread, and its abundance seems to reflect the overall composition of the planet earth, in particular the earth's crust, that is so rich in silicates. But in fact quartz is very unevenly distributed among different geological environments: some rocks are completely void of quartz, in others quartz is the main component.
Later we will see that if you look at the earth as a whole - or even just the earth's crust - it is very remarkable that there is quartz at all. Its presence is the result of a complex chain of geological processes that lead to a differentiation of the earth's mantle material into a wide range of different crustal rocks.
Overall Composition of the Earth's Crust
If one looks at the frequency of chemical elements, it is obvious that almost the entire earth's crust is made of silicates. Figure 1 shows a pie chart of the proportions of the 10 most frequent elements for the earth's crust.
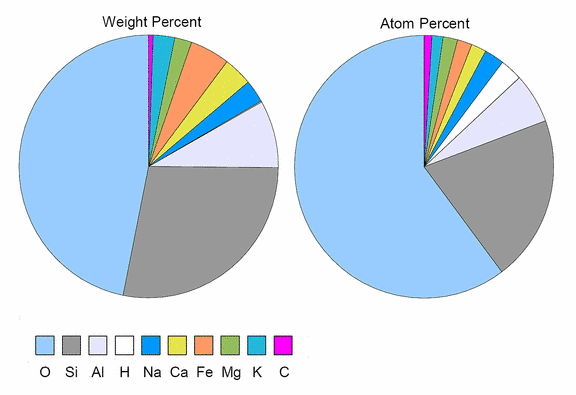
Fig.1: The 10 Most Frequent Chemical Elements of the Earth's Crust
The left pie chart shows the relative proportions measured as weight or mass percent, while the right pie chart shows atom percents, a more meaningful measure of the number of atoms of each element. Note that while hydrogen makes only an insignificant contribution to the overall mass, its influence on rock chemistry is more important, as can also be seen from its relatively high proportion of atoms.
Almost two thirds of all atoms in the earth's crust are oxygen atoms, and almost one quarter of them are silicon atoms. These two elements comprise more than 80% of the atoms and almost 75% of the mass of the earth's crust. All oxygen is either bound in metal and non-metal oxides or in "salts" of oxygen acids like carbonic acid, phosphoric acid, sulfuric acid, or - the largest group - silicic acid. The reverse is also true: almost all metal atoms are bound in oxides or silicates.
Silicates are "salts" of orthosilicic acid, H4SiO4, and contain single or connected SiO4 tetrahedra, metal and sometimes non-metal elements. It is possible to view silicates as the products of silica (SiO2) and metal oxides, as silica is the acid anhydride of orthosilicic acid:
Because of this relation, it was once common practice to express the chemical composition of a silicate mineral as relative proportions of silica and metal oxides: KAlSi3O8 (orthoclase feldspar) can be translated into K2O•Al2O3•6SiO2, for example.
Silica and Classification of Rocks
Silica has a very important chemical property: When it reacts with metal oxides, it can be incorporated into the new compound as individual SiO44- groups, but it might also form chains, rings, sheets and networks of SiO4 tetrahedra. The composition of the silicates that form in such a reaction strongly depends on the amount of silica added to the mixture. Compare that to other anions like the carbonate ion CO32-, chloride Cl-, or sulfate SO42-, that do not form stable chains or networks. For example, adding an extra amount of sulfuric acid in a reaction with Ca(OH)2 will not result in a different product, the extra amount will simply not react:
2 H2SO4 + Ca(OH)2 → Ca2SO4 + 2 H2O + H2SO4 [14]
If one puts twice the amount of quartz into the mixture, a different mineral will form, enstatite, containing chains of connected SiO4 tetrahedra:
In nature reactions that actually involve metal oxides are uncommon, and silicates typically react with each other and other compounds to form new silicates. And, of course, the reactions that take place when minerals form in a natural solidifying magma are far more complex. But the principle still holds: The mineral composition of an igneous rock is strongly dependent on the relative amount of silica in the magma that it formed from.
For example, only igneous rocks that originate from a magma with more than about 50 weight-% silica can contain feldspars, and only igneous rocks with more than about 63-65% silica can contain quartz as a rock-forming component.
As silica is the anhydride of an acid, rocks that are rich in silica are called acidic rocks, and those with a low silica content are called basic rocks. This classification is based on the amount of both free silica (quartz) and bound silica in silicates (as SiO4). So a rock that is entirely made of orthoclase feldspar (KAlSi3O8) will be called silica rich and acidic, as it contains 65% silica. A peridotite rock, composed largely of olivine, (Mg,Fe)2SiO4, is called basic, as it contains only about 30-40% silica.
... to be continued ...
 Printer Friendly Version
Printer Friendly VersionCopyright © 2005-2013, A.C. A k h a v a n
Impressum - Source: http://www.quartzpage.de/gen_ori.html
