last modified: Monday, 16-Jul-2012 00:30:24 CEST
Document status: almost complete
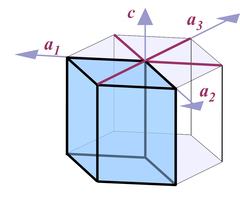
A crystal can be viewed as being composed of different regular geometrical bodies. These basic bodies are the forms of the crystal (see the Basic Terms section for an explanation).
Of the many possible crystallographic forms of quartz, about 40 can be found in nature. Each of the forms is given a descriptive name, a specific scientific numerical symbol that reflects its relation to the crystal lattice, and the corresponding faces on the crystal are given a specific greek or latin letter. Most of the forms (about 30) can rarely be observed, so I will focus mainly on those that are common and important. But even from these few forms a surprisingly large number of different shapes can be derived.
Basic Crystal Forms of Quartz
The following table lists the most important crystallographic forms in quartz. A more detailed description for each of them is given below. A comprehensive list of quartz crystallographic forms can be found in Rykart, 1995, along with an estimate of their abundance.

H264-movie, 256x256px: left_quartz.mp4 367kb |
The different faces correspond to different crystal lattice planes. They can be related to different forms, and accordingly the whole crystal can be viewed as the intersection of these forms. The rendered quartz crystal, for example, is the intersection of 5 forms: r, z, m, s, and x.
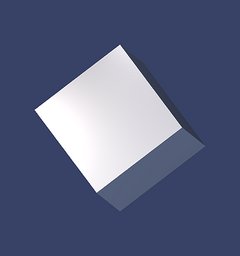
Rhombohedron - r and z
There are two common rhombohedral forms in quartz crystals, the positive rhombohedron r and the negative rhombohedron z. These forms, in particular the rhombohedron r, occur in almost all quartz crystals. The corresponding crystal faces are called the r-face and the z-face.
H264-movie, 256x256 px: form_r.mp4 166kb |
To the right you can see the r-rhombohedron or r-form, the next picture shows a z-rhombohedron or z-form.

H264-movie, 256x256 px: form_z.mp4 161kb |

H264-movie, 256x256 px: form_r+z.mp4 277kb |
The shape of a crystal can be viewed as an intersection of forms. An intersection of bodies corresponds to the volume that all bodies share. In the right figure you would "cut off" everything that only belongs to one rhombohedron and you would get the intersection, in this case a hexagonal bipyramid.

H264-movie, 256x256 px: form_r+z_trans.mp4 256kb |
Quartz crystals tend to grow a bit faster on the z faces than the r faces. The first guess would be that because of that the z faces are larger, but the opposite is true. Growth occurs perpendicular to a face, so if one face grows faster than another, then its surface actually shrinks. Thus the r-faces on quartz crystals are usually larger, and sometimes z-faces are completely absent. Accordingly, the intersection of the r- and z-forms in quartz crystals is usually not a perfect hexagonal bipyramid but shows trigonal symmetry. In crystals that are twinned after the Dauphiné law r- and z-faces cannot be distinguished and the faces of the pyramid tend to be sized more equally.
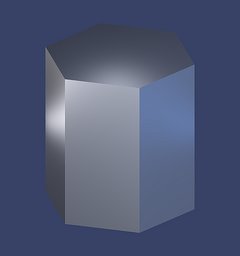
Hexagonal Prism - m
H264-movie, 256x256 px: form_m.mp4 192kb
|
The hexagonal prism is actually an open form that only consists of the prism "walls", called m-faces. Its faces lie parallel to the walls of the quartz unit cell, as indicated in the figure below the rendering, with the unit cell tinted blue. In the figure and in the computer rendering it is terminated at its top and bottom by the so-called c-face, corresponding to the basal plane or basal pinacoid (see below for an introduction).
Striation
The m-face very often shows a characteristic horizontal striation (perpendicular to the c-axis), a very distinctive feature of quartz crystals that helps identifying them even when crystal tips are missing. Often you read that these little steps in the prism faces are caused by rhythmic growth driven by external factors. This is not true, however.
First, one should keep in mind that striations are also observed in other minerals, tourmaline group minerals and pyrite being good examples, but no one suggests that these are caused by fluctuations in the environmental conditions. The idea that it is related to changes in growth speed in quartz is based on the fact that the steps run perpendicular to the direction of fastest growth, and that many quartz crystals tend to narrow towards the tips, but in fact rhythmic changes in the preferred habit should not lead to striations or narrowing. If striation in quartz was caused by external factors, it would be difficult to explain that it is usually missing completely on other crystal faces.
Second, one would expect to see similar step patterns on quartz crystals from the same pocket, but this is not the case, the striations do not even match on different m-faces of the same crystal.
The strongest argument against an external cause of striations comes from experiments with industrially grown quartz. This quartz is grown under steady conditions in NaOH solutions and normally does not show striation. Instead, the m-faces show asymmetric growth hillocs on the surface (NaOH solution is chosen because it gives the high growth rates needed for a commercial production), which sounds as if the proponents of an external cause were right. But industrially grown quartz does develop striations when it is grown in a NaCl solution at relatively low speed (Hosaka and Taki, 1978, in Miyata et al., 1989). "Relatively low speed" means relative to the desired growth rates for industrial production - it is still very fast compared to growth under most natural conditions. From studies of fluid inclusions in quartz crystals it is known that a NaCl solution is much closer to natural brines than a NaOH solution. So it is an inherent property of the prism faces to develop striation under most natural conditions.
Quartz crystals with certain growth forms sometimes lack striation. It is often absent on skeleton quartz and scepter quartz crystals. The striation is generally less pronounced on small crystals.
Basal Pinacoid - c
The basal pinacoid, also called the basal plane is a common form in most minerals, but the corresponding face, the c-face, is extremely rare in quartz. Its presence is perhaps only caused by corrosive processes. The website Micro-Taunus presents amethyst crystals with a small c-face found at the Four Peaks Mine, Arizona. It has been shown that the faces from this locality have developed by dissolution on surfaces that were first mechanically broken and overgrown (Kawasaki et al., 2006). Similar faces are seen on ametrine crystals from Bolivia, and these also seem to have developed by a dissolution process.Calling this form "basal" is somewhat redundant, that notion is already contained in the term pinacoid.
Trigonal Bipyramid - s

H264-movie, 256x256 px: form_s.mp4 140kb |
The specimen on the second image from Amputofiamendia, Madagascar, shows an unusually large, almost diamond-shaped s-face.

10mm 1007x1520 98kb - 2014x3039 303kb |

0mm 1340x986 242kb - 2679x1971 692kb |
Trapezohedron - x

x-form H264-movie, 256x256 px: form_x.mp4 129kb |
The positive trapezohedron is the characteristic form of quartz, it reflects the symmetry properties of quartz crystals: 3-fold rotational symmetry around the c-axis, 2-fold rotational symmetry around the a-axes, and lack of mirror symmetry. The presence of this form is what makes quartz a member of the trigonal-trapezohedral crystal class, with the according Hermann-Maugin symbol 3 2. The trapezohedron shows enantiomorphy, that is, it can be left- or right-handed, just like quartz crystals. The x-face is also the best crystal face to determine if a crystal is right-handed, left-handed, or twinned.
The characteristics of the form can much better be grasped when a trapezohedron is wider than the very slim x-form, so there are two other images and movies for demonstration.
|
The form looks a bit like a badly twisted rhombohedron, one could say either twisted left- or rightward. In both movies the forms rotate clockwise, and they are illuminated from the left. Nevertheless the forms appear to be mirror images of each other. Once again, both "wide" trapezohedra in the left and right image are different from the slim x-form, and are only shown as an example of what trapezohedra look like.
The corresponding crystal face of the trapezohedron x is called x-face and, interestingly, very often manifests as a simple triangle at the upper left or right corner of an m-face. Although this is difficult to estimate, I would say it is even more rare than the s face. At some locations, like the Central Alps in Switzerland, it is quite common, however.
There is also a negative trapezohedron, and a corresponding -x face, ( { 1 6 5 1 } for right and { 1 5 6 1 } for left-handed crystals), that is found at the same position as the positive trapezohedron, but under the z face. It is much rarer than the positive trapezohedron.
The x-face seems to be indicative of very slow crystal growth. Like the s-face, it is unlikely to be found on amethyst, ferruginous, pink, milky quartz, or generally any quartz with a high content of impurities.

10mm 750x960 102kb - 1500x1920 352kb |

10mm 800x1004 83kb - 1600x2008 280kb |
The crystal shows a number of irregular lines or "cracks" that run vertically through the frontal m- and x-face, so called sutures. Often you read that these indicate twin boundaries in a crystal, but as you can see, this is clearly not so (more on this can be found in the chapter Twinning).

10mm 1000x1000 103kb - 2000x2000 290kb |
These crystals also carry s-faces, visible as relatively dark narrow triangular faces left to the large rhombohedral faces. Their shape deviates from the typical diamond-shape because the hexagonal prism gets narrower to the tip. The specimen is from the Kullu Valley, Himachal Pradesh, India.

20mm 1007x1520 193kb - 2014x3039 660kb |
Accessorial faces like the x- or s-face do not necessarily assume their "ideal" position at the rhombohedral face at the tip, but can also be found on the prism faces: there are 2 chlorite-covered x-faces at the left side and 1 at the right side of prism of the large crystal that look like triangular indentations.
Steep Rhombohedra

H264-movie, 256x256 px: form_M.mp4 129kb |

10mm 1242x1007 159kb - 2484x2014 537kb |

10mm 840x1006 113kb - 1680x2012 369kb |

10mm 1024x1496 180kb - 2048x2992 532kb |

10mm 1007x1488 206kb - 2014x2976 589kb |

5mm 660x924 101kb - 1320x1848 321kb |
Other Forms

5mm 1000x1424 149kb - 2000x2848 448kb |
Further Information, Literature, Links
To get a good overview of the many crystal forms and habits of quartz, Rudolf Rykart's Quartz Monographie is the best source I know, but it's written in German.The standard book in English language is C. Frondel's introduction to Silica Minerals.
In the Internet, Marino Bignami's site www.faden.it is a very good source, albeit in Italian.
 Printer Friendly Version
Printer Friendly VersionCopyright © 2005-2013, A.C. A k h a v a n
Impressum - Source: http://www.quartzpage.de/crs_forms.html