last modified: Sunday, 12-Jan-2014 22:43:21 CET
Document status: construction site
Overview of Silica Polymorphs
Quartz is just one of 11 crystalline and 2 non-crystalline polymorphs[1] (also called modifications) of the compound silica, SiO2. 12 of these modifications can be found in nature, and 11 of them on Earth. All silica minerals[2] are united in the silica group according to Dana's classification, and in the quartz group[3] according to Strunz's classification.SiO2 Polymorphs | |
|---|---|
|
Silica Polymorphs (Network Silicates) Quartz, Low-Quartz, α-Quartz, Alpha-Quartz High Quartz, β-Quartz, Beta-Quartz α-Tridymite, Low-Tridymite β-Tridymite, High-Tridymite α-Cristobalite, Low-Cristobalite β-Cristobalite, High-Cristobalite Moganite (Lutecite, Lutecine) Coesite Keatite
|
Non-Silica Polymorphs Stishovite Seifertite
|
|
Non-Crystalline Mineraloids Opal (contains water), with 2 microcrystalline and 2 non-crystalline variants Lechatelierite, Silica Glass
| |
|
Related Compounds Melanophlogite (not pure SiO2) Chibaite, IMA2008-067 (not pure SiO2) Silhydrite (not pure SiO2, contains crystal water)
| |
The basic structural element of silica is the SiO4 tetrahedron. Quartz consists of interconnected SiO4 tetrahedra that build up a rigid three-dimensional network (discussed in detail in the chapter Quartz Structure). There are many possible ways of connecting SiO4 tetrahedra different from that found in quartz, realized in various other silica polymorphs. Since all of them consist of a three-dimensional SiO4 network, all are classified as network silicates.
Stishovite and Seifertite are special cases because they are not made of SiO4 tetrahedra and accordingly are not classified as a network silicates. Instead, each silicon atom is surrounded by 6 oxygen atoms, and the packing of atoms is much more dense.
Dependence of Structure on Temperature
Of all silica polymorphs, quartz is the only stable form at normal ambient conditions, and all other silica polymorphs will - given sufficient time - eventually transform into quartz. The other polymorphs are stable at different and sometimes very special conditions, mostly high temperatures and high pressures, but some of them may also form at low temperatures and pressures under conditions where quartz is stable.In theory, at normal pressure trigonal quartz (α-quartz) will transform into hexagonal β-quartz at 573°C, upon further heating the SiO2 will transform into hexagonal β-tridymite at 870°C and later to cubic β-cristobalite at 1470°C. At 1705°C β-cristobalite finally melts:
| 573°C | 870°C | 1470°C | 1705°C | |||||
|
α-Quartz trigonal 2.65 g/cm3 |
 |
β-Quartz hexagonal 2.53 g/cm3 |
 |
β-Tridymite hexagonal 2.25 g/cm3 |
 |
β-Cristobalitecubic 2.20 g/cm3 |
 |
Silica Melt |
However, tridymite does usually not form from pure β-quartz, one needs to add trace amounts of certain compounds to achieve this (Heaney, 1994). So the β-quartz-tridymite transition is skipped and the sequence looks like this:
| 573°C | 1050°C | 1705°C | ||||
|
α-Quartz trigonal 2.65 g/cm3 |
 |
β-Quartz hexagonal 2.53 g/cm3 |
 |
β-Cristobalitecubic 2.20 g/cm3 |
 |
Silica Melt |
The changes in crystal structure lead to changes in the specific density: an increasing temperature corresponds to increasing vibrations of the atoms in the crystal lattice, and as these need more and more space, more open crystal structures are favored. So why don't the atoms form an open structure in the first place? Because the structure must also be in accordance with constraints on the geometry of the covalent bonds, in particular the angled Si-O-Si bond that connects SiO4 tetrahedra.
As long as the temperature changes very slowly, the whole process is fully reversible.
But things get far more complex when the temperature is increased or decreased more quickly. If one heats up a quartz crystal very quickly, it will still undergo a phase transition to β-quartz, but the β-quartz will then "skip" the transition to β-cristobalite and directly melt at a much lower temperature, at 1550°C.
| 573°C | 1550°C | |||
| α-Quartz |  |
β-Quartz |  |
Silica Melt |
It makes sense that β-quartz has a lower melting point: it is less stable than β-cristobalite at that temperature and its crystal lattice is more easily broken up. So it doesn't really make sense to say that quartz melts at 1705°C, because low quartz never melts, and because the melting temperature depends on how quickly you raise the temperature.
However, this process is not reversible. Instead, if a silica melt is cooled quickly, its liquid structure will be preserved and it will turn into amorphous silica glass, called lechatelierite when found in nature. There is no well defined melting point for silica glass which slowly turns into a very viscous liquid upon heating. It is often said (and I've written this before, too) that silica glass is an extremely viscous liquid, just like ordinary window glass, but both glasses are considered as regular solids.
| 1000 - 1500°C | ||
| Silica Glass |  |
Silica Melt |
Even more strange is what happens to silica glass that is heated up: one would expect it to be converted to β-quartz, β-tridymite or β-cristobalite, depending on the temperature. But in fact it will simply turn into β-cristobalite, just as silica melt would.
| ca.1000°C | 1705°C | |||
| Silica Glass |  |
β-Cristobalite |  |
Silica Melt |
This conversion is of technical importance in the industrial production of silica glass, as great care has to be taken to avoid the formation of cristobalite crystals within the glass.
If the polymorphs β-tridymite and β-cristobalite are cooled quickly below the respective transition temperatures, their crystal structure is first preserved until they will transform into polymorphs with closely related structures, α-tridymite and α-cristobalite, at 114°C and 270°C, respectively:
| 114°C | ||
| α-Tridymite |  |
β-Tridymite |
| 270°C | ||
| α-Cristobalite |  |
β-Cristobalite |
The transition is fully reversible even at relatively quick temperature changes, just like the transition of α-quartz to β-quartz.
At low pressures there are actually 3 groups of silica polymorphs each of which has 2 closely related members: one low-temperature member given an α-prefix, and one high-temperature member of the same name, but with a β-prefix. Some authors prefer a low-prefix and a high-prefix.
| Low Pressure Silica Polymorphs | |||||
|---|---|---|---|---|---|
| high- or β-polymorph stable at metastable at crystal system Si-O-Si angle |
β-Quartz 573°C - 870°C - hexagonal 153° |
β-Tridymite 870°C - 1470°C 117°C - 870°C hexagonal 180° |
β-Cristobalite > 1470°C 270°C - 1470°C cubic 151° |
||
| low- or α-polymorph stable at metastable at crystal system Si-O-Si angle |
α-Quartz < 573°C - trigonal 144° |
α-Tridymite - < 117°C triclinic 140° |
α-Cristobalite - < 270°C tetragonal 147° |
||
During the transition from a α- to a β-variant the atoms in the crystal lattice only get slightly displaced relative to each other, but they don't change places inside the crystal lattice (the topology is preserved). Because these α-β-transitions are only based on alterations of the angles and the lengths of the chemical bonds, they take place instantaneously. Such a phase transition is generally called displacive, as it only requires relative displacements of the atoms without the need to break chemical bonds. Because the angular Si-O-Si bonds get straightened out, the high-temperature silica polymorphs all possess a higher symmetry than their low-temperature counterparts (hexagonal > trigonal > triclinic; cubic > tetragonal).
All the other transitions of one silica polymorph into another (like from β-quartz to β-tridymite) require the chemical bonds to be broken up and reconnected to alter the crystal structure. Accordingly, such a transition is called reconstructive. In general, complete reconstructive transitions between polymorphs need a lot of time. Quick changes in temperature do not allow for the complete rebuilding of the crystal structure, and the transition will be skipped. This is what happens when β-quartz directly melts at 1550°C without transformation into β-tridymite, and what happens if β-tridymite and β-cristobalite get cooled very quickly. β-tridymite and β-cristobalite can exist outside the temperature range at which they are stable, but as they will very slowly alter to another polymorph that is more stable at those conditions, they are called metastable polymorphs.
Phase Diagram of Silica Polymorphs
Figure 1 shows the temperature and pressure conditions at which SiO2 polymorphs are stable in a so-called phase diagram of SiO2. A complete phase diagram would also show the conditions where SiO2 forms a gas, above 2477°C at normal pressures, but since I don't have data on pressure dependence for that area, this temperature range is omitted.
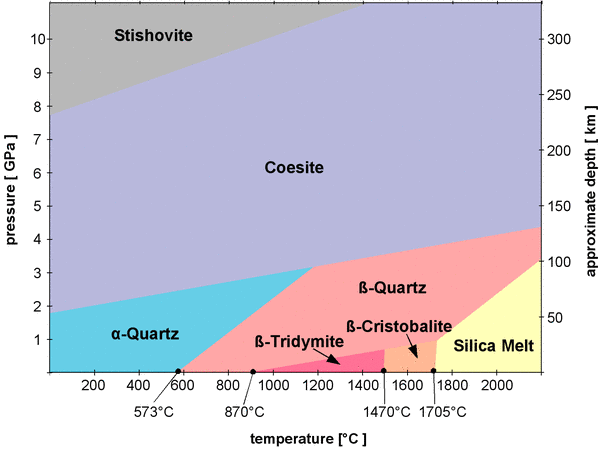
Fig.1: Phase diagram of silica
Data from:
➛Hollemann and Wyberg, 1985
➛Wenk and Bulakh, 2003
➛Rykart, 1995
The phase diagram in Fig.1 does not contain all SiO2 polymorphs.
Most of the phase boundaries (the borders between the areas) are inclined to the right. For example, quartz will transform into β-quartz at 573° at normal pressures, but the transition temperature quickly increases with pressure. At a pressure of 2 GPa (Giga pascal, 109 pascal) β-quartz forms at about 1000°C. The rising temperature increases the vibrations of the atoms so they need more space, but the external pressure compresses the crystal lattice and counteracts the effect of temperature[4].
α-quartz, β-quartz, β-tridymite, and β-cristobalite have already been introduced as low pressure polymorphs, while coesite and stishovite are called high pressure polymorphs and are not stable at normal pressures. Coesite and stishovite have a higher density than the low pressure polymorphs, in particular stishovite, which has a specific density of 4.29 g/cm3. While Coesite is still made of interconnected SiO4 tetrahedra, stishovite assumes a completely different arrangement of atoms in its crystal lattice. In all low pressure polymorphs as well as in coesite, the silicon atoms are surrounded by 4 oxygen atoms. The silicon in them is said to have a coordination number of 4. This is also the case in all silicate minerals. The coordination number of stishovite is 6, so the silicon atoms are all surrounded by 6 oxygen atoms. The crystal structure of stishovite does not fit in the classification scheme of silicates, so stishovite could well be considered a non-silicate. Stishovite and coesite are both not stable at normal pressures and normal temperatures, but since their transitions into a low pressure silica polymorphs are reconstructive phase transitions that involve a complete rearrangement of the atoms in the crystal lattice, both are metastable at normal conditions.
α-quartz is not stable above 3 GPa and not stable at temperatures above 1200°C, so its stability field occupies only a small part in the phase diagram. It looks as if it might be stable at normal pressures and temperatures and thus accumulate at the Earth's surface, but it might not be that abundant in the varying geological environments inside the Earth's crust and mantle. Where can we expect to find the various polymorphs inside the Earth? To answer that question, the left y-axis of the diagram in Fig.1 is a scale of the pressure, and the right y-axis shows a scale of depth in the Earth's mantle and crust that corresponds to the pressures (it is only a rough estimate, as the pressure does not increase perfectly linear with depth and also varies locally). That way one can superimpose a diagram of the pressure and temperature conditions (p-T conditions) of the Earth's crust and upper mantle, as shown in Fig.2.
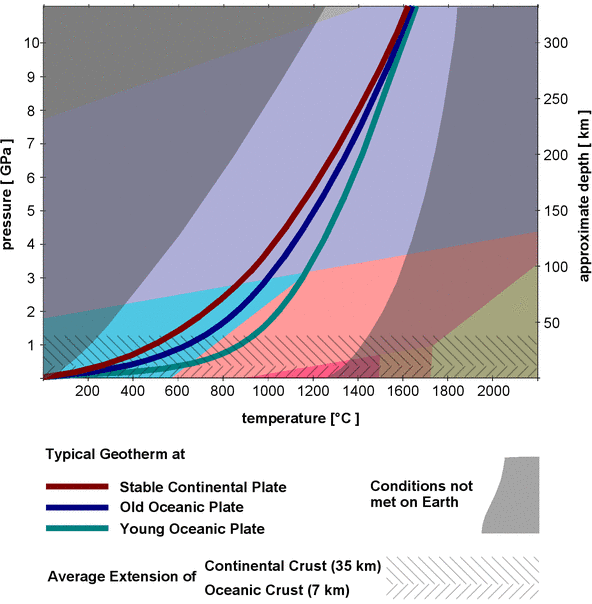
Fig.2: Geotherms and natural P/T conditions superimposed on the phase diagram of silica
Data from:
➛Fowler, 2004
➛Okrusch and Matthes, 1995
➛Skinner and Porter, 1987
➛Markl, 2004
The change of temperature with depth is plotted as a so-called geotherm[5], the rate at which the temperature changes is called the geothermal gradient, usually given as degrees Kelvin per 100 m. The geothermal gradient is not the same everywhere, and it normally decreases with depth, that is, the temperature raises first quickly at shallow depths and then more and more slowly at greater depths.
The possible p-T conditions found on Earth are confined to a narrow corridor between 2 gray-shaded areas in Fig.2. Typical geotherms for stable continental, young and old oceanic crust are shown as solid colored lines. The bottom line of the diagram along the x-axis corresponds to conditions at the surface, and the temperature range for rocks at the surface is between approximately -100°C (in the arctic) and 1300°C (in hot volcanic magma[6]). The conditions at the left border of the corridor are found when crust is quickly subducted at plate boundaries, whereas the conditions at the right border are met in quickly rising magma, for example, at volcanic eruptions.
Fig. 2 also shows the average thickness of oceanic (7km) and continental crust (35km). Continental crust shows large variations in thickness: Where continental plates collide, it may be up to 90km thick, in rift zones it might be just 10km.
Judging from the geotherms, α-quartz will be the stable form of silica in the entire continental and oceanic crust under normal conditions. Only where hot magma is pushed up quickly one might expect to find β-quartz. If a silica-rich crust gets subducted to great depth, the α-quartz in it will not be transformed to β-quartz, even if the temperature exceeds 573°. Silica glass will never form from a silica-rich magma, and β-cristobalite is also very unlikely to form. Of course it is still a different matter if α- or β-quartz will actually form under certain p-T conditions inside the Earth, as the presence of free silica is mainly determined by the chemical composition of the rock.
α-Quartz or Low Quartz
α-quartz (=alpha quartz) or low quartz is the specific name of "ordinary" quartz. The "low" refers to the fact that this is the stable silica polymorph at low temperatures as opposed to the very similar β-quartz that is stable only beyond 573°C.β-Quartz or High Quartz
Also see Tables of Properties below.β-quartz (=beta quartz) or high quartz is a high temperature polymorph of silica with a crystal structure very similar to that of quartz. At normal pressures quartz undergoes a structural transition to β-quartz at 573°C. The relative positions of the atoms in the crystal lattice get shifted slightly, each atom remains surrounded by the same neighbors, and none of the chemical bonds in the structure get broken up for this change to happen, so the transition is displacive. The relative positions of the atoms within the SiO4 tetrahedra remain almost identical and the tetrahedra are not distorted. Instead, the SiO4 tetrahedra get slightly twisted in a way that causes the crystal structure to assume a hexagonal symmetry.
Like ordinary low quartz, β-quartz occurs in left- and right-handed crystals. Interestingly, a Dauphiné twin of low quartz that is heated above 573°C and cooled again will assume the same geometry of twin domains. This is remarkable because Dauphiné twinning cannot occur in a hexagonal crystal. Lattice defects that cause the twinning and determine the spatial distribution of twin domains apparently survive the heating procedure and initiate the formation of Dauphiné twinning again after cooling.
The transition from low to β-quartz is immediate and reversible, that is, the structural change happens instantaneously as soon as the temperature is reached, and is reversed to trigonal low quartz structure upon cooling under the critical temperature. Thus, all β-quartz specimen in collections are in fact low-quartz pseudomorph after β-quartz. The term used for the special case of pseudomorphs of one polymorph after another is paramorphosis.
Because β-quartz is only stable at temperatures that are well beyond that of hydrothermal watery solutions, it is typically formed in cooling igneous silica rich magmas. In most cases, the quartz grains in intrusive igneous rocks like granite are irregular, only in rare cases the crystal developed their ideal shape. In extrusive igneous (that is, volcanic) rocks well-formed beta quartz crystals are more common. It can also be found in well formed crystals in gas cavities of silica rich volcanic rocks, a famous location of amethyst colored barrel-shaped hexagonal crystals are the hills Colli Euganei near Padova in Northern Italy (Sovilla 1997, Prüfer 2005, www.faden.it). Transparent β-quartz is rare, most crystals are translucent and have a dull surface.

5mm 800x880 83kb |
Moganite
Also see Tables of Properties below.This silica polymorph has first been described by Flörke et al., 1976, in volcanic rocks of the Mogan formation on Gran Canaria island, Spain. It later turned out to be identical with lutecite, a so-called length-slow chalcedony type that was commonly found in chalcedony. Moganite is typically intergrown with cryptocrystalline quartz to form chalcedony. Its internal structure is much like that of a quartz that is intensely twinned according to the Brazil law on the unit cell scale, and the quartz crystallites in chalcedony are usually also polysynthetical Brazil law twins, being composed of alternating layers of right- and left-handed quartz (see description of chalcedony in the chapter Types of Quartz).
The moganite content of different chalcedony specimen and agates varies greatly and may reach around 85%, but is typically between 1% and 20% in agate, chalcedony, chert and flint (Heaney and Post, 1992). Very rarely "pure" moganite is found, Götze et al., 1998, mention a specimen with 97% moganite from Gran Canaria. The formation of moganite seems to be promoted in cherts that have been formed as evaporites ("Magadi-type cherts"), here the concentrations are generally higher (30-50%). The amount of moganite seems to decrease with time as it is slowly converted into chalcedony, and agates older than approximately 100-150 million years seem to be almost void of it.
Moganite is not represented in the silica phase diagram, and is essentially a meta-stable silica polymorph. Apparently the other silica polymorphs cannot be converted to melanophlogite by changes in pressure or temperature.
Opal
Also see Tables of Properties below.In Angloamerican literature, opal is usually not considered a mineral because it is amorphous and varies considerably in composition, but because it has some degree of homogeneity, it is called a mineraloid.
Most people consider opal as rare because they think of the gem varieties, but in fact opal is fairly common in many low-temperature environments. Opal forms from silica rich watery solutions and, very similar to chalcedony, watery silica gels. It can be found in cracks of silica rich volcanic rocks, but also in sedimentary rocks. In volcanic rocks it may form on rock walls from silica transported with water steam (Flörke et al., 1973). Most opal is of biogenic origin, however, as it forms the opaline skeletons of single celled algae and marine plankton. The skeletons of these organisms accumulate as sediments at the ocean floor and get transformed into diatomites and radiolarites.
Opals differ considerably in their microstructure and composition, their common denominator is the overall non-crystalline structure and their water content. Apart from that, opals are further classified (Flörke et al., 1991; Graetsch, 1994) as
-
Opal-C - microcrystalline opals made of cristobalite, sometimes called lussatine
Transitional state in the formation of diatomites and radiolarites from oplaine skeletons. Found in nodular concretions in sediments, as so-called menilite
-
Opal-CT - microcrystalline opals made of intergrown cristobalite and tridymite, sometimes called lussatite
Found in common opal, as well as a transitional state in the formation of diatomites and radiolarites from opaline skeletons. Found in opaline concretions in sediments
-
Opal-AG - amorphous opals with a gel-like structure
This is the structure of potch opal, precious opal and fire opal.
-
Opal-AN - amorphous opals with a network-like structure
A common example is hyalite or glass opal.

10mm 1200x1120 204kb - 2400x2240 617kb |
Precious opal is opal-AG, amorphous opal made of tiny stacked silica spheres. The silica spheres are left over from the gel state, and their size is in the same order of magnitude as the wavelength of light, with a diameter of approximately 100 - 500 nm. The voids between the spheres are filled with silica and water. If the spheres are all of the same size and stacked regulary, they act as a diffraction grating on the light and cause the strong opalescence, because water and silica have different refractory indices (Sanders, 1964). If the water in the voids is lost, the opalescence and the colors may vanish.

10mm 1440x1000 259kb - 2880x2000 720kb |

10mm 1040x1056 137kb - 2080x2112 405kb |

10mm 1360x1024 220kb - 2720x2048 619kb |
α-Cristobalite and β-Cristobalite
Also see Tables of Properties below.α-cristobalite is a constituent of many opals, but it is quite rare as an individual mineral. Well-formed crystals are only found in cavities of silica-rich volcanic rocks. Although its crystal structure is tetragonal, it is commonly found as pseudocubic octahedral crystals, because it did not form directly, but as a paramorph after cubic β-cristobalite, the high-temperature polymorph. Because the crystal structures of both polymorphs do not differ very much, the crystal shape is well preserved.
Cristobalite is a rock-forming mineral, and occurs as a transitional silica polymorph in the form of opal-C (opal made of tiny cristobalite spheres) during the diagenesis of sediments made of opaline skeletons of marine organisms. It will slowly be converted into quartz (chalcedony or microquartz) with time. Before this conversion is completed, these biogenic sediments rich in opal-A and opal-C are sometimes called porcellanites.

0.5mm 1024x992 133kb |

10mm 2048x1424 1024kb - 4096x2848 2812kb |

1mm 1280x944 296kb - 2560x1888 804kb |
Cristobalite often looks milky because of numerous tiny cracks inside the crystals.

1mm 1024x864 156kb - 2048x1728 504kb |

10mm 1744x1504 221kb - 3488x3008 590kb |
Tridymite
Also see Tables of Properties below.Various studies have reported that tridymite will undergo several subtle structural changes upon heating, accompanied by changes in symmetry and crystal class. Different classification schemes for these various tridymite structures exist (for a discussion, see Heaney, 1994), but because the tridymite crystals found in nature are all α-tridymite, I do not present all the different structural varieties.
Like cristobalite, tridymite is more common as a component of opal (in particular opal-CT) than as individual mineral and only found as low-temperature polymorph α-tridymite, paramorph after β-tridymite. Like cristobalite, tridymite formed at high temperatures maintains the shape of the hexagonal high-temperature form, and accordingly looks hexagonal, despite its triclinic crystal structure.

1mm 1473x983 170kb |
Lechatelierite
Also see Tables of Properties below.Lechatelierite is the name of natural silica glass that formed by rapid cooling of molten silica. Glass is an amorphous substance, and thus lechatelierite is technically not a mineral.
Under normal geologic conditions lechatelierite does not form. The temperatures found in the Earth's crust are too low to melt quartz sand, and even if there was a quartz melt, the cooling process would also be too slow to allow for an amorphous glass to form.

approx. 100mm 1376x912 406kb - 2752x1824 1362kb |
Lechatelierite can also be found at impact craters of meteorites, should the meteor strike at silica-rich rocks.
Melanophlogite
Also see Tables of Properties below.Strictly spoken, melanophlogite is not a silica polymorph, as it always contains a small amount of organic compounds that seem to stabilize its structure and are required for the formation of the mineral. It is found in crevices of sedimentary rocks. Because of its open cage-like molecular structure melanophlogite is sometimes considered as a relative of zeolites, or is classified as a so-called clathrasil (Higgins, 1994).
Melanophlogite was first found in the sulfur mines of Racalmulto, Agrigento and Lercara, Palermo, both in Sicily, and described by von Lasaulx in 1876 (Skinner and Appleman, 1963). A second locality was found in Chvaletic, Czech Republic (Žák, 1972) in a metamorphosed ore deposit in volcanogenic sediments. A similar occurrence in altered serpentines is reported from Mt. Hamilton, California (Cooper and Dunning, 1972). Meanwhile the best known locality is Fortulino, Livorno, Italy, where it is found in crevices of altered serpentine rocks.

2mm 1280x886 183kb - 2560x1772 524kb |

1mm 1500x1000 194kb |
Chibaite (IMA2008-067)
Chibaite has been accepted as a valid mineral species by the I.M.A. in 2009, and was catalogued as IMA2008-067. Like melanophlogite, chibaite is, stricktly spoken, not a silica polymorph, because it always contains a small amount of hydrocarbons and is not pure silica. It is the natural analogon of a class of synthetic compounds called dodecasil 3C or MTN. Like melanophlogite it is a clathrasil with a cage-like structure made of SiO4 tetrahedra. It does not contain sulphur, just hydrocarbons. So far the only locality is Arakawa, Chiba Prefecture, Japan, where it is found in cracks in weakly metamorphosed marine sedimentary rocks, along with calcite and chalcedony.
Tables of Properties
The following tables give an overview of the properties of SiO2 polymorphs. They include opal and melanophlogite, which are somewhat special cases and should not be treated as a separate silica modifications. I've also added silhydrite, which contains water like opal, but in a fixed ratio.
Data from various sources, among them:
Rykart 1995
Hollemann & Wiberg, 1985
Wenk & Bulakh, 2003
Rösler, 1991
Heaney, 1994
Higgins, 1994
Hemley, Prewitt, Kingma, 1994
Physical Properties | ||||
|---|---|---|---|---|
| Name | Color | Specific Density |
Mohs Hardness |
Refractive Indices |
| α-Quartz | colorless | 2.65 | 7 | 1.54422 - 1.55332 |
| β-Quartz | colorless | 2.53 | - [7] | 1.53 - 1.54 |
| α-Tridymite | colorless | 2.35 | 6½ - 7 | 1.471 - 1.488 |
| β-Tridymite | colorless | 2.25 | - [8] | 1.469 - 1.473 |
| α-Cristobalite | colorless | 2.33 | 6½ | 1.485 - 1.487 |
| β-Cristobalite | colorless | 2.27 | - [9] | 1.479 |
| Moganite | gray | 2.55 | ? | |
| Coesite | colorless | 3.01 | 7½ | 1.594 - 1.599 |
| Stishovite | colorless | 4.29 | 7½ - 8 | 1.799 - 1.826 |
| Seifertite | 4.31 | > 8 | ||
| Keatite | gray | 2.5 | ? | 1.513 - 1.522 |
| Opal | colorless any color |
1.9 - 2.5 | 5½ - 6 | 1.43 |
| Lechatelierite | colorless | 2.20 | 6½ | 1.46 |
| Melanophlogite | colorless, brown, yellow |
2.02 | 5½ - 6 | 1.425 - 1.457 |
| Chibaite | colorless | ? | ? | ? |
| Silhydrite | white | 2.116 | 1 | 1.466 |
Chemical Properties | |||||
|---|---|---|---|---|---|
| Name | Formula | Stability at Normal Conditions |
Coordination Number of Si |
Si-O Distance |
Si-O-Si Angle |
| α-Quartz | SiO2 | stable | 4 - tetrahedral | 0.161 nm | 144° |
| β-Quartz | SiO2 | instable | 4 - tetrahedral | 0.162 nm | 153° |
| α-Tridymite | SiO2 | metastable | 4 - tetrahedral | 0.154 nm -0.171 nm |
140° |
| β-Tridymite | SiO2 | instable | 4 - tetrahedral | 0.153 nm -0.155 nm |
180° |
| α-Cristobalite | SiO2 | metastable | 4 - tetrahedral | 0.158 nm -0.169 nm |
147° |
| β-Cristobalite | SiO2 | instable | 4 - tetrahedral | 0.151 nm | 151° |
| Moganite | SiO2 | stable - metastable | 4 - tetrahedral | ||
| Coesite | SiO2 | metastable | 4 - tetrahedral | ||
| Stishovite | SiO2 | metastable | 6 - octahedral | ||
| Seifertite | SiO2 | instable | 6 - octahedral | ||
| Keatite | SiO2 | ? | 4 - tetrahedral | ||
| Opal | SiO2 • n H2O | metastable | 4 - tetrahedral | ||
| Lechatelierite | SiO2 | stable | 4 - tetrahedral | ||
| Melanophlogite | SiO2 • n (C,H,O,S) | metastable | 4 - tetrahedral | ||
| Chibaite | SiO2 • n (CH4, C2H6, C3H8, C4H10) |
? | 4 - tetrahedral | ||
| Silhydrite | 3SiO2 • H2O | ? | 4 - tetrahedral | ||
Occurrence | ||
|---|---|---|
| Name | Abundance | Geological Environment |
| α-Quartz | abundant | in sedimentary, metamorphic and many igneous rocks at temperatures below 573°−1000°C |
| β-Quartz | never at the Earth's surface | in the Earth's crust at temperatures above 573°−1000°C |
| α-Tridymite | crystals rare, common constituent of dense silica forms | Silica rich volcanic rocks, component of some opals |
| β-Tridymite | rare (?), never at the Earth's surface | |
| α-Cristobalite | crystals rare, common constituent of dense silica forms | Silica rich volcanic rocks, component of some opals, deep sea sedimentary rocks, porcellanites |
| β-Cristobalite | rare, never at the Earth's surface | |
| Moganite | very common | never pure, intergrown with quartz in chalcedony |
| Coesite | rare | rarely captured within crystals of other minerals in mantle xenoliths; more common in impact craters |
| Stishovite | very rare | in impact craters, possibly abundant in the Earth's lower mantle |
| Seifertite | very rare | discovered in the Shergotty Martian meteorite, possibly in the Earth's lower mantle |
| Keatite | artificial (very rare?) | artificial (in stratospheric dust particles?) |
| Opal | very common | Precipitates at low temperatures in silica rich watery solutions, also biogenic origin |
| Lechatelierite | rare | As so called fulgurites, formed when quartz sand has been molten by a stroke of lightning |
| Melanophlogite | very rare | At sulfur deposits; in cracks in weathered serpentinites and associated sediments |
| Chibaite | very rare | Crevices in sedimentary rocks |
| Silhydrite | very rare | |
Crystallographic Data | ||||
|---|---|---|---|---|
| Name | Crystal System |
Crystal Class | Hermann -Mauguin Symbols |
Space Group |
| α-Quartz | trigonal | trigonal - trapezohedral |
3 2 | P3121 (left hand) P3221 (right hand) |
| β-Quartz | hexagonal | hexagonal - trapezohedral |
6 2 | P6422 (left hand) P6222 (right hand) |
| α-Tridymite | triclinic | triclinic - pedial |
1 | F1 |
| β-Tridymite | hexagonal | dihexagonal - dipyramidal |
6/m 2/m 2/m | P63/mmc |
| α-Cristobalite | tetragonal | tetragonal - trapezohedral |
4 2 2 | P 41212 |
| β-Cristobalite | cubic | hexoctahedral | 4/m 3 2/m | Fd3m |
| Moganite | monoclinic | monoclinic - prismatic |
2/m | I2/a |
| Coesite | monoclinic | monoclinic - prismatic |
2/m | C 2/c |
| Stishovite | tetragonal | ditetragonal - dipyramidal |
4/m 2/m 2/m | P 4/mnm |
| Seifertite | orthorhombic | orthorhombic - dipyramidal |
2/m 2/m 2/m | Pbcn |
| Keatite | tetragonal | tetragonal - trapezohedral |
4 2 2 | P43212 |
| Opal | macroscopically amorphous |
- | - | - |
| Lechatelierite | amorphous glass | - | - | - |
| Melanophlogite | tetragonal | ditetragonal - dipyramidal |
4/m 2/m 2/m | P 41/nbc |
| Chibaite | cubic | isometric - diploidal |
m3 (2/m 3) | Fd3 |
| Silhydrite | orthorhombic | ? | ? | ? |
Further Information, Literature, Links
A nice overiew of silica polymorphs and related structures, both natural and artificial, is given in the book"Silica - Physical behavior, geochemistry and materials applications", by P.J. Heaney, C.T. Prewitt and G.V. Gibbs, in particular in the articles by Heaney (1994), by Higgins (1994) and by Hemley, Prewitt and Kingma (1994)
Footnotes
1 The polymorphs or modifications of a substance have they same chemical formula but different crystal structures. Graphite and diamond are polymorphs of carbon.2 That excludes stishovite and seifertite which should be considered as oxides as they are not made of SiO4 tetrahedra.
3 That includes stishovite and seifertite.
4 If the density of a substance increases with temperature, the opposite happens, as it is the case for water and ice. Ice has a lower density than water, and around its freezing point, external pressure can cause it to melt again.
5 A geotherm is inferred from various data and models on heat generation and heat transport, and not measured directly.
6 Only at the impact of a large meteorite higher temperatures and pressures occur at the Earth’s surface.
7 Not stable below 573°C
8 Not stable below 870°C
9 Not stable below 1470°C
 Printer Friendly Version
Printer Friendly VersionCopyright © 2005-2013, A.C. A k h a v a n
Impressum - Source: http://www.quartzpage.de/gen_mod.html
